Regulatory Science & Product Development Experts helping customers
Commercialise Products | Reduce Time to Market | Achieve Compliance | Grow Business
Phyto Pharmaceuticals
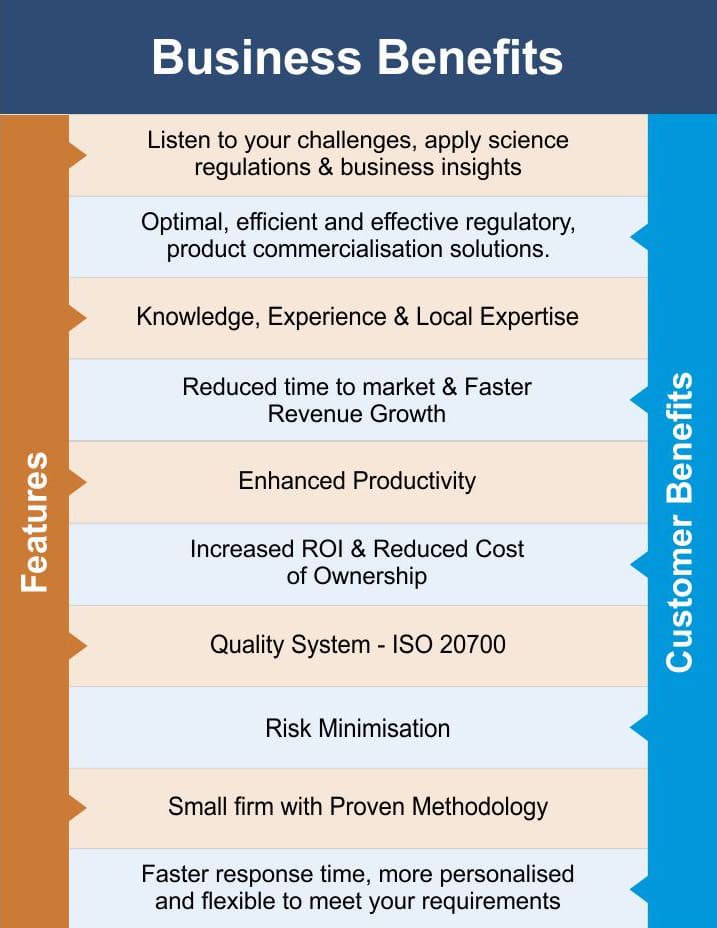
Vyomus Consulting is a regulatory and development partner helping biopharmaceutical companies commercialize Phyto Pharmaceuticals in India. From discovery to commercialization, we design and implement efficient and effective regulatory and product development solutions which integrate science, regulations and business objectives, to overcome product commercialisation challenges.
CDSCO designates a product to be a Phyto Pharmaceutical if a substance is purified and the fraction is standardised, assessed qualitatively and quantitatively with defined minimum four bio-active or phytochemical components of an extract of a medicinal plant or its part, for internal or external use in human beings or animals for diagnosis, treatment, mitigation or prevention of any disease or disorder but does not include drugs administered through a parenteral route.
In brief, there is a set procedure of application submission, product testing, registration dossier submission, review and approvals from various stakeholders that are required to facilitate Product Approval / Market Authorisation.
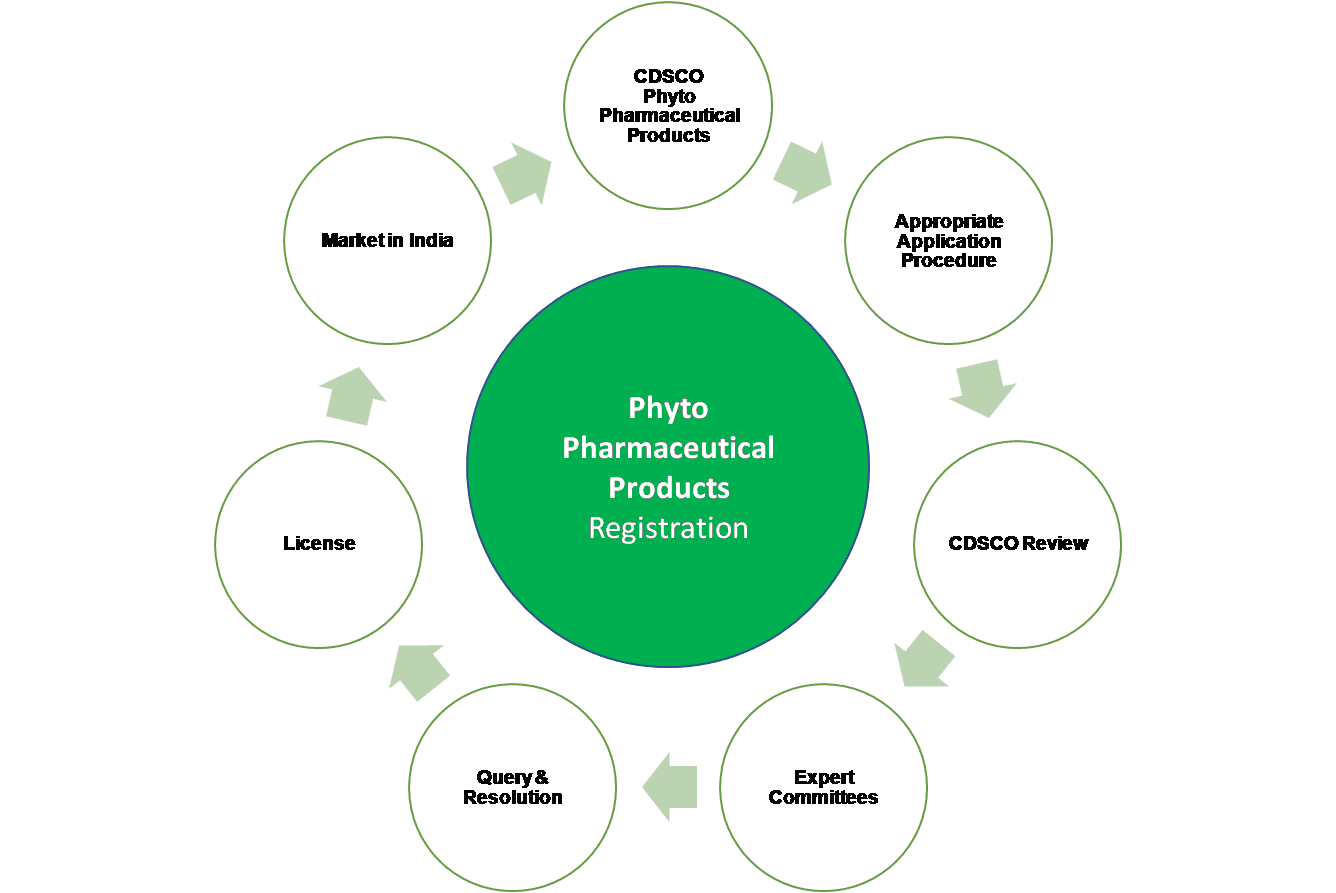
Vyomus Consulting provides end-to-end Product Registration Services as well as Authorised Agent Services to all importers and manufactures of Phyto Pharmaceuticals. Our service offerings will help you import, manufacture, test, conduct clinical trials and distribute your products in India (Market Authorisation), efficiently so as to achieve faster product approvals leading to enhanced access to Markets and Opportunities.
Our senior practitioners have a unique blend of business, science, regulations and technology expertise and include experts in product development, regulatory affairs, pharmacology, toxicology, preclinical and clinical trial design and evaluation, biostatistics, each with extensive product commercialisation experience.
Regulatory Consulting, Regulatory Strategy, Preclinical Study Approvals, Clinical Study Approvals, Market Authorisations, Labelling Services, Regulatory Submissions, Regulatory Reporting, Regulatory Intelligence, Authorised Agent Services.