Regulatory Science & Product Development Experts helping customers
Commercialise Products | Reduce Time to Market | Achieve Compliance | Grow Business
Medical Devices& In-Vitro Diagnostics
Vyomus Consulting is a regulatory and development partner helping Medical Devices and In-Vitro Diagnostics (IVD) companies commercialize their products in India. From ideation to commercialization, we design and implement efficient and effective regulatory and product development solutions which integrate science, regulations and business objectives, to overcome product commercialisation challenges.
In order to legally import and market or manufacture medical device/s and or IVD’s in India, a manufacturer needs to be compliant to Indian regulations and legislation. In summary, medical device & in vitro diagnostic medical devices manufacturers who wish to import and market their products in India, have to register their manufacturing site/s and product/s through their registered office in India, or their Subsidiary or through an Importer or an India based Authorised Agent.An organisation which proposes to manufacture a medical device or IVD product in India will have to register itself through CDSCO.
Only one Indian Authorized Representative office is needed for the whole country. Vyomus Consulting will represent you legally in India so that all your distributors can buy the products from us, although activities like distributor identification, product marketing, product sales etc., are not being supported at present.
Even though Schedule R and R1 of the Drugs & Cosmetics Act 1940 & Rules 1945 regulated a very limited number of products (primarily condoms, mechanical contraceptives, perfusion sets, Hypodermic syringes and needles), Medical device specific regulations are quite young in India. The year 2005 was when a limited number of products(Notified Medical Devices) were brought under regulatory control. These products were being regulated as Drugs rather than as Medical Devices under the Drugs & Cosmetics Act & Rules.
From 1st January 2018, a comprehensive set of rules called the “Medical Device Rules 2017” were specifically framed and implemented for Medical Devices & IVD’s under the Drugs & Cosmetics Act & Rules to regulate the medical devices industry with relevant and applicable regulations.
The Medical Devices Rules 2017 comprises of 12 Chapters, 8 Schedules and 40 Forms. It has been drafted to ensure its as comprehensive as possible within present day industry limitations and is designed to be flexible enough to add or delete the list of categorized products which are to be published from time to time by Central Drugs Standard Control Organisation (CDSCO).
Classification of medical devices and in vitro diagnostic medical devices follow an internationally accepted, risk based approach. Based on the level of anticipated risk for the intended use, both, medical devices and in vitro diagnostic medical devices are classified either as :
- Low risk – Class A;
- Low Moderate risk – Class B;
- Moderate High risk – Class C and
- High risk – Class D.
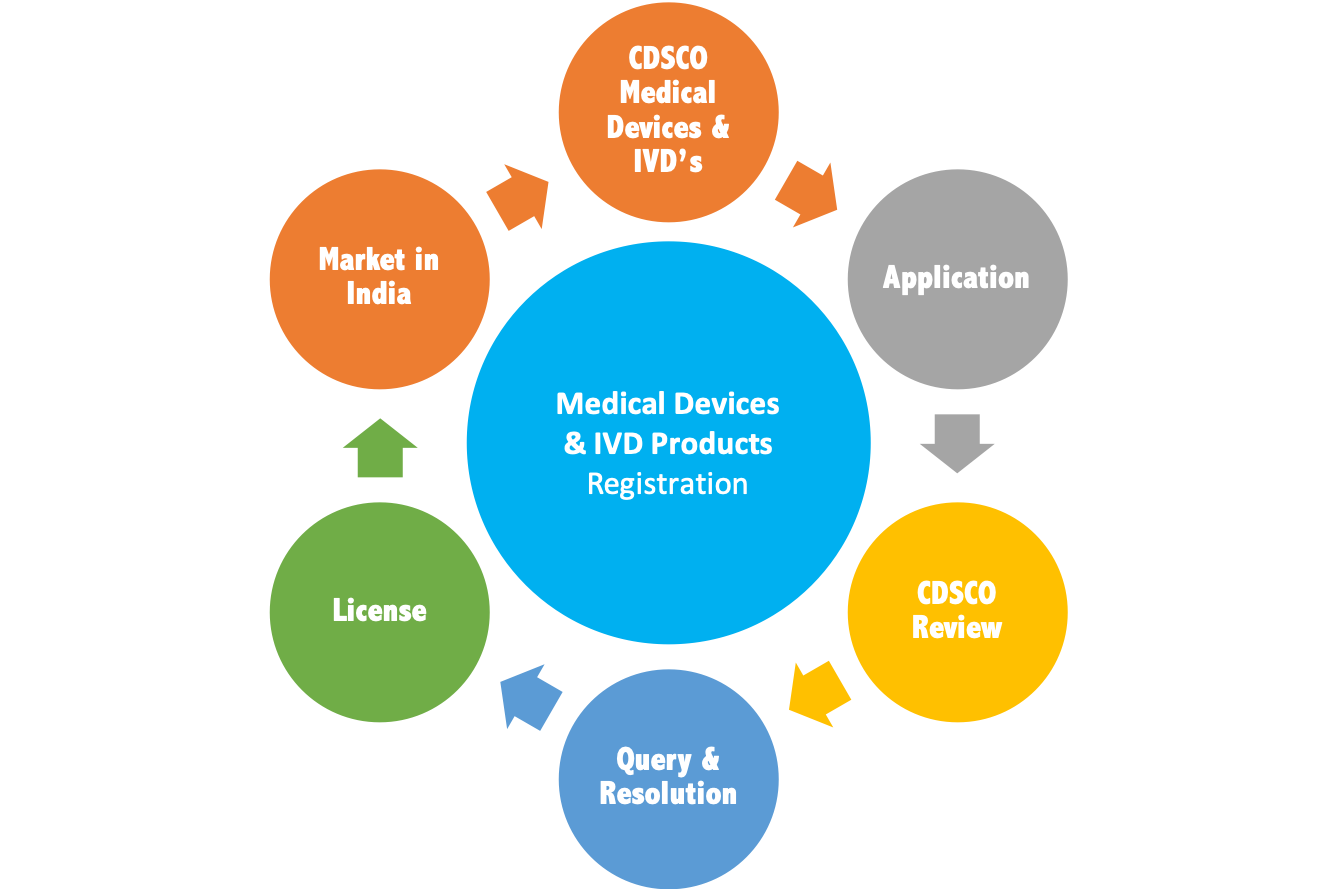
Vyomus Consulting provides end-to-end Product Categorisation Services,Product Registration Services as well as Authorised Agent Services to all importers and manufactures of Medical Devices & IVDs. Our service offerings will help you import, manufacture, test, conduct clinical trials and distribute your products in India (Market Authorisation), efficiently so as to achieve faster product approvals leading to enhanced access to Markets and Opportunities.
We provide “intelligent strategies“ instead of simplified, often cost-intensive routine Device Development Programs to ensure the highest possible regulatory acceptance of your product. We thereby ensure effective compliance and thus help you, our customers navigate the complex, medical devices regulatory landscape and make right decisions. Hence, emerging and established medical device firms trust Vyomus Consulting as their partner to deliver customized strategic guidance and creative “hands-on” solutions to categorize and register their products efficiently.
Our senior practitioners have a unique blend of business, science, regulations and technology expertise and include experts in product development, regulatory affairs, pharmacology, toxicology, preclinical and clinical trial design and evaluation, biostatistics, each with extensive product commercialisation experience.
Regulatory Consulting, Regulatory Strategy, Preclinical Study Approvals, Clinical Study Approvals, Market Authorisations, Labelling Services, Regulatory Submissions, Regulatory Reporting, Regulatory Intelligence, Authorised Agent Services.

