Regulatory Science & Product Development Experts helping customers
Commercialise Products | Reduce Time to Market | Achieve Compliance | Grow Business
Authorized Agent Services
In order to legally register, import and market, sell your regulated products in India you need to be compliant to Indian regulations and legislation. When a company does not have a legal business, registered and physical office with statutory personnel and required licenses in India, a legal India Authorized Representative / Agent with the above needs can be formally appointed. This Authorized Representative will also be held responsible for pre-certification and post-market surveillance inquiries.
Vyomus Consulting will represent your organization as your official Indian Authorized Representative / Agent in accordance with India applicable regulatory legislation.Only one Indian Authorized Representative office is needed for the whole country. Vyomus Consulting will represent you legally in India without any commercial conflict of interest activities like product distribution, product marketing, product sales etc.
Many product distributors are happy to take on the role of an official Indian Authorized Representative. This relationship would curtail the manufacturer’s ability to work with multiple distributors in India or change distributor/s due to performance challenges.
Product registration licenses and Marketing Authorisations of the manufacturer areissued in the name of the appointed India Representative as identified by the manufacturer. If a distributor is appointed as the authorized representative, this relationship cannot be changed at least till the duration of the licenses or authorisations. Also, this can become a commercial challenge if there were to be an opportunity or a requirement to switch distributor/s.If there were a requirement to switch distributors, the registration process has to be initiated all over again.
Vyomus Consulting is a regulatory and product development consulting organization, which specializes purely in the regulated products space and provides independent in-country representative services. Since, the India Authorized Representative is legally responsible for your products in India, it is extremely important to be represented by a professional firm that specializes in providing regulatory services and whose interests do not conflict with that of your company.
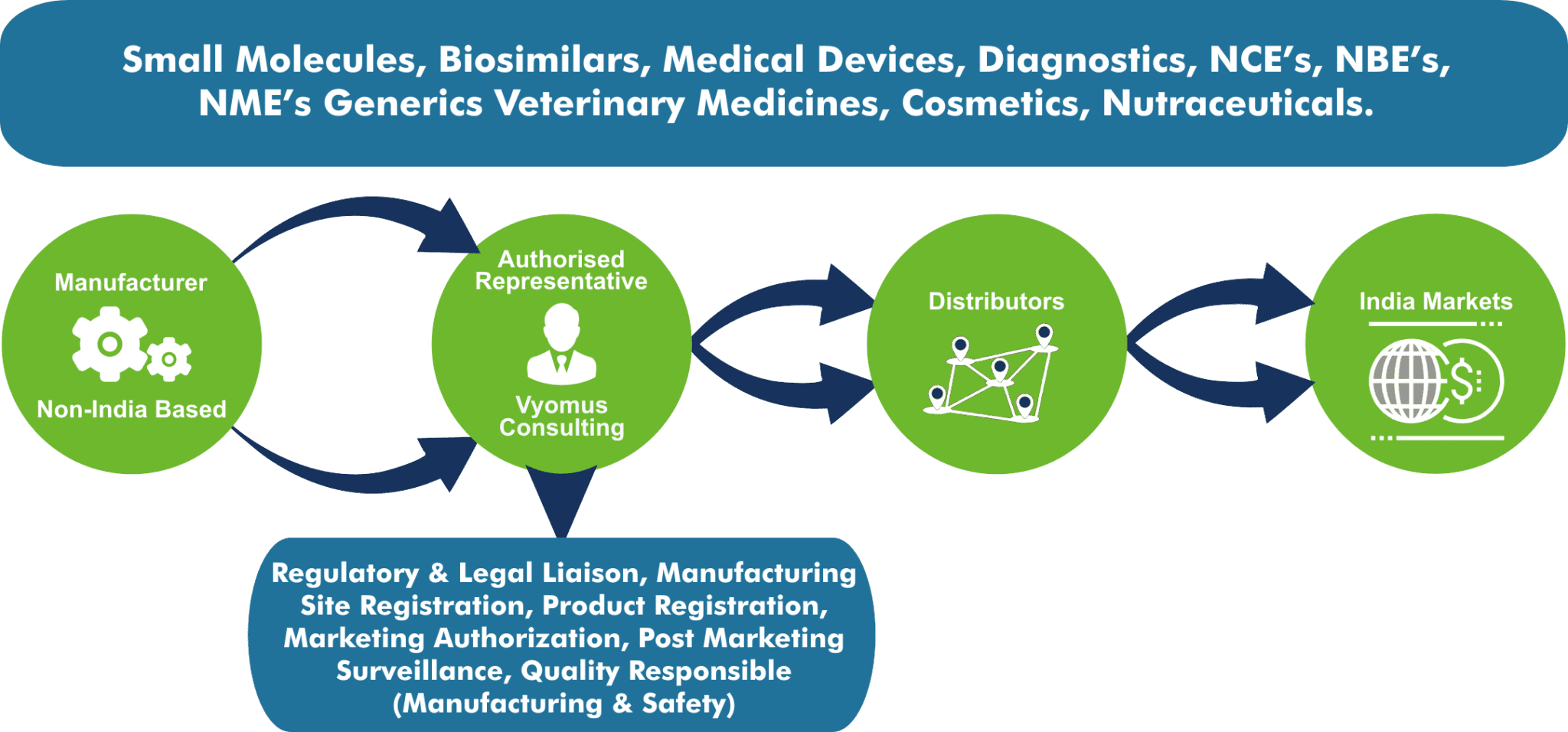
An India Authorized representative is responsible for product registrations and for acting as the legal liaison between the manufacturer, based outside India and Indian regulatory agenciessuch as CDSCO (Central Drugs Standard Control Organization) etc.,
In addition to being a resident or registered commercial organization of India, the India Authorized Representative must have the required statutory infrastructure and licenses along with prior experience in the industry. The India representative has significant responsibilities and is also legally liable for the product in India according to applicable product specific regulations. The extent of responsibility is very different from the role of the “US Agent” representative required by the United States FDA.
The India Authorized Representative, through a legal Power of Attorney executed by the manufacturer, is authorized to submit product registration documents and act as a point of contact for any inquiries related to the product by the regulator. The Representative is also involved in vigilance activities and acts on the manufacturer’s behalf if an onsite inspection of the manufacturer’s facility is required.
- Provide authorization to place our name, local mailing address and phone/fax numbers on your product submissions and registrations to the CDSCO;
- Upon request by the CDSCO provide information about your products, and names and addresses of Indian distributors;
- Assist in coordination of an inspection if CDSCO selects your manufacturing site for an inspection/audit.
Industry focus :Pharmaceuticals, Medical Devices, Cosmetics & Wellnes, Food & Nutrition.
- Market Authorisations, Clinical Study Approvals, New Drug Approval, Labelling Services, Regulatory Strategy, Regulatory Reporting, Regulatory Submissions, Regulatory Intelligence, Authorised Agent Services, Market Access.
- Post marketing services including Complaint handling, Adverse incident reporting and product recall support as part of Product safety reporting, Query management, PSUR Writing & Submission activities
- All statutory Marketing Authorization holder’s requirements.

