Regulatory Science & Product Development Experts helping customers
Commercialise Products | Reduce Time to Market | Achieve Compliance | Grow Business
Product Development Advisory
Vyomus Consultingunderstands the science behind your product, applicable regulations and expected business outcomes, to help customers formulate and implement development strategies for Pharmaceuticals and Medical Devices, right from lead candidate/prototype selection to final regulatory submissions leading to commercialisation. Our local knowledge combined with global standards and scientific regulatory expertise will ensure success by adding effectiveness, efficiency and manageability to your product development programs.
Vyomus Consulting developed and well tested implementation methodology – “ASIA” generates significant business gains on all implemented projects. It integrates science, regulations and measurable business outcomes, at each stage of the product development lifecycle to ensure a focus on results for both business and functional stakeholders. The business consulting approach and impeccable process implementation and delivery practices ensure that optimal product development solutions are implemented to achieve specified business objectives, strategically.
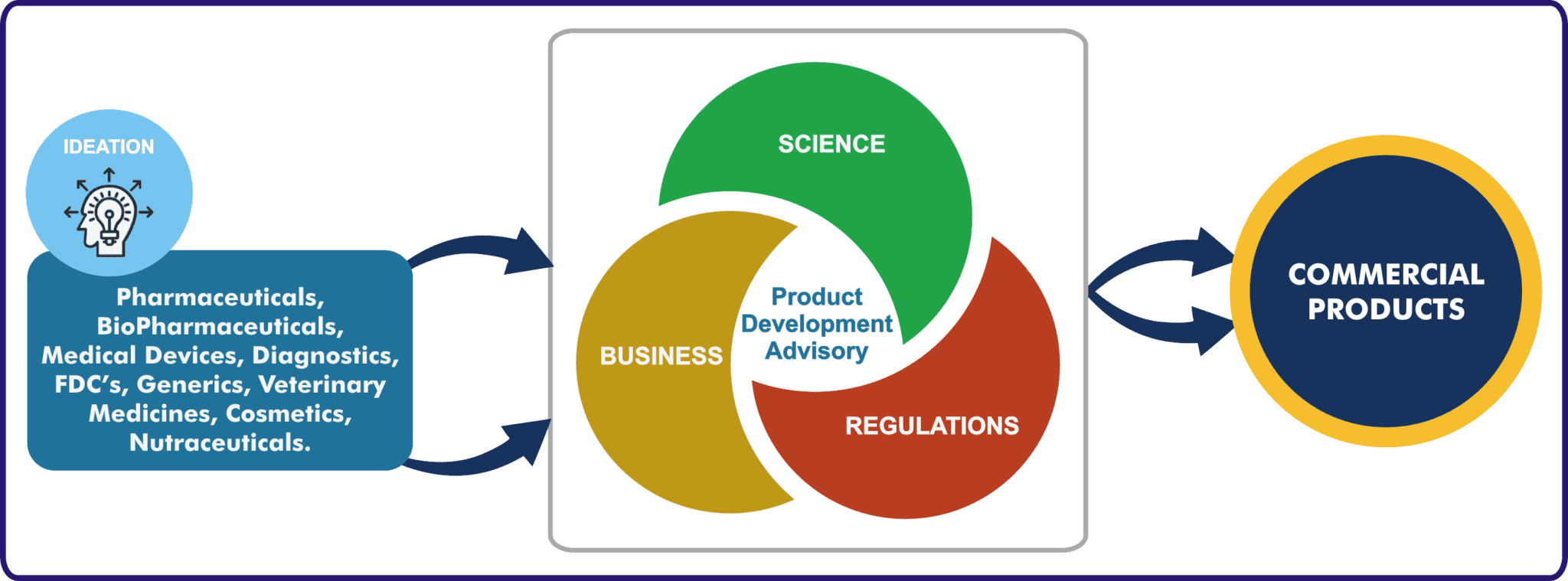
Industry focus : Pharmaceuticals, Medical Devices& IVDs.
Product Development Strategy & Plan; Project Management; Supplier/Vendor Selection & Qualification
| <===== Integrated Program Delivery =====> |
|---|
| Business Focus | Science Focus | Regulatory Focus |
|---|---|---|
| Portfolio Analysis | Scientific Advice | Regulatory Strategy & Advice |
| Market Assessment | Comprehensive Product Development Strategy and Implementation Plan |
Regulatory Services to fulfilPreclinical, Clinical Trial and Market Authorisation Submission Requirements |
| Due Diligence, Gap Analysis – Candidate Selection |
Comparative Effectiveness Strategy and Clinical Research |
Submissions: NDA, ANDA, MAA, BLAs. |
| <=== Development Execution ===> |
|---|

