Regulatory Science & Product Development Experts helping customers
Commercialise Products | Reduce Time to Market | Achieve Compliance | Grow Business
Labelling Services
Vyomus Consulting offers a full range of professional services to centrally manage the entire label life cycle – from initial design & review to obsolescence for the Medicine, Medical Device, Cosmetics, Food & Nutritional industries.
For the Pharmaceuticals, Medical Devices, Cosmetics & Wellness, Food & Nutrition products , we provide labelling strategies and scientific, regulatory content, design, develop Product Labels for Commercial Sale, Product Labels for Import, Product Labels for Export, Clinical Study Labels, Investigational Product Labels, Products for Testing Labels, Core Data Sheets (CCDS), Summary of product characteristics (SmPC), Safety Data Sheets (SDS) etc.,
We also offers complete protocol breakdown, regulatory review, and consultation for clinical studies. We have also partnered with a globally recognized translation company to provide translation services in this space. These services coupled with our industry leading labelling methodology can reduce clinical label cycle times from months to just a few weeks.
Vyomus Consulting is highly experienced in developing and reviewing all labelling documentation for India, US and EU markets, in full accordance with currentregulations. This includes core data sheets, Summary of Product Characteristics, Patient Information Leaflets, inner and outer package labels and bar coding practices.
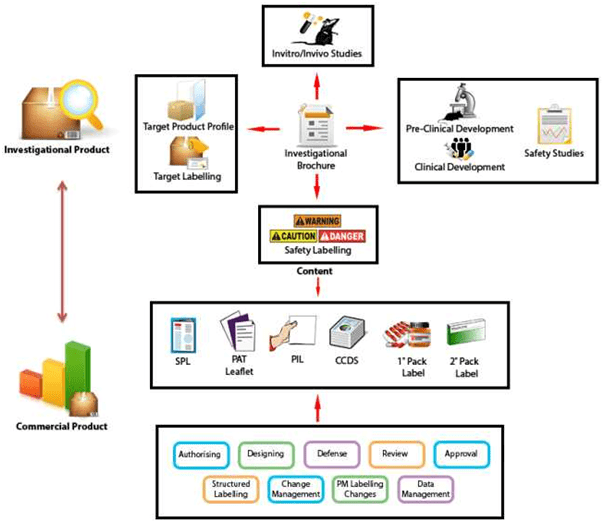
Vyomus Consulting also provides XML Structured Product Labelling for the Pharmaceutical Industry. Structured Product Labelling (SPL) is an XML standard used by the United States Food and Drug Administration (FDA) to facilitate the communication of drug labelling data reliably among various groups such as hospitals, doctors, pharmacies, and the general public. SPL is both an HL7- and ANSI-approved standard.
Industry focus :Pharmaceuticals, Medical Devices, Cosmetics & Wellnes, Food & Nutrition.
- Development of Product Labels for Commercial Sale, Product Labels for Import, Product Labels for Export, Clinical Study Labels, Investigational Product Labels, Products for Testing Labels etc.,
- Authoring and designing effective and defensible labelling content and format implementation before filing
- Authoring or updating, development of Investigational Brochure,Core Data Sheets (CCDS), Summary of product characteristics (SmPC), Safety Data Sheets (SDS, formerly known as MSDS)and development of Target Product Profiles and Target Labelling
- Assistance in case of regulatory requests to correct non-compliant,submittedlabelling content
- Supporting regulatory/Labelling, pharmacovigilance, medical, clinical, marketing and legal departments.
- Labelling decision making structure for development products and post marketing Labelling changes.

