Regulatory Science & Product Development Experts helping customers
Commercialise Products | Reduce Time to Market | Achieve Compliance | Grow Business
Product Development Strategy
Developing new products or modifying existing products so they appear new, and offering those products to current or new markets is the definition of product development strategy. There is nothing simple about the process. It requires keen attention to competitors and customer needs now and in the future, the ability to finance prototypes and manufacturing processes, and a creative marketing and communications plan.
Regulated products such as Pharmaceuticals, Medical Devices &IVDs, Cosmetics, Food & Nutritionals are required to follow a structured development plan to demonstrate their safe, usability for a particular therapeutic, beautification or health challenge.Bringing products that your customers want to market at the right time and at the lowest cost is the most basic goal of product development. Yet with limited budgets, people, and time, many organizations are hard-pressed to streamline their product development processes, despite the fact that doing so has repeatedly proven to advance that very goal.
Also, quite a number of organisations spend their time putting out fires and pursuing projects aimed at catching up to their competitors. They have far too many projects going on at once and all too often seriously overcommit their development resources. They spend too much time dealing with short term pressures and not enough time on the strategic mission of product development. A more efficient and productive way would be to rationalise the product development portfolio along with developing and implementing a Product Development Plan.
The essential goal of a product plan should be to ensure that a product is built that delivers some business value to a specific set of customers in order to meet certain financial goals based upon a defined corporate strategy. Successive plans should increase that product’s effectiveness in doing so. A product plan describes the market opportunity, profiles the target customers, specifies product, pricing, identifies the financial goals, indicates the key priorities for development and enhancement, and provides a roadmap for delivery for at least the next four quarters.
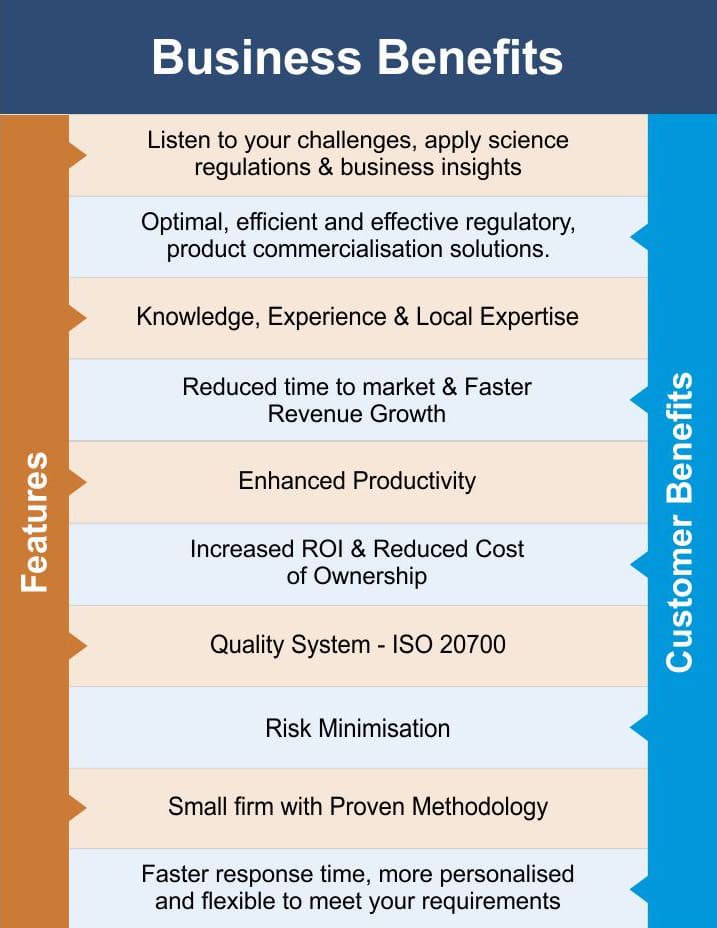
Vyomus Consulting consists a team of experienced, senior regulatory & development experts who provide national and international Product Development strategy and advice and help customers propel pharmaceuticals, biologics, medical devices / combination products, Cosmetics, Food & Nutritionals over early stage hurdles, past key milestones, and on to first-cycle approvals. Our senior practitioners are expert in regulations, formulation development and testing, pharmacology/toxicology, pharmacokinetics, biostatistics, program management, and preclinical, clinical trial design and evaluation.
From discovery to commercialization, we design, implement and provide solutions specifically tailored to your business challenges which ensures significant return on investment and reduces total cost of ownership. Integrating Science, Regulations and Measurable Business outcomes, combining local knowledge with global standards, our team moves beyond providing expert advise to get your project off the ground and moving at a fast pace.
Our overall experience includes over six hundred preclinical, clinical trial approvals, product registrations, marketing authorizations, import and marketing authorizations, manufacturing and marketing authorization and transactions covering a large number of therapeutic and product categories.
Industry focus :Pharmaceuticals, Medical Devices, Cosmetics & Wellnes, Food & Nutrition.
- Scientific Assessment, Regulatory Assessment, Regulatory Intelligence.
- Create, Maintain, UpdateProduct Development Plans.
- Create, Maintain, UpdateRegulatory Roadmaps.
- Create, Maintain, UpdateRegulatory Strategy.
- Create, Maintain, UpdateRisk & Mitigation Plans.